- The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number.
- The mass of the atom of a particular isotope relative to hydrogen 1 (or to one twelfth the mass of carbon 12), generally very close to the whole number represented by the sum of the protons and neutrons in the atomic nucleus of the isotope; it is not to be confused with the atomic weight of an element, which may include a number of isotopes in natural proportion.
Phone Number (Toll-Free): (781) 849-5555. FAQs Who can play the lottery in the state of Massachusetts? Players must be 18 years old or older to purchase Massachusetts Lottery tickets. If an adult purchases the winning ticket and claims it in the name of a person under the age of 18, the director can direct payment to the minor.
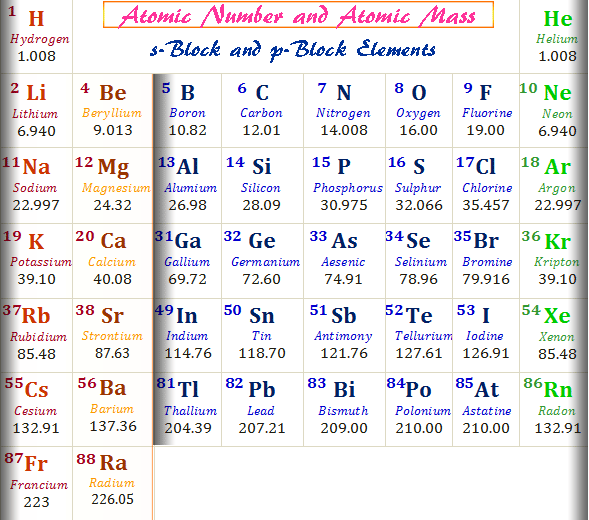
What is mass number equal to?

1 Answer
The total number of nucleons - protons and neutrons - in the nucleus of an atom is called the Mass Number.

For example, the most common isotope of fluorine has an atomic number of 9 and a mass number of 19.
The atomic number tells us there are 9 protons in the nucleus (and also 9 electrons in the shells surrounding the nucleus).

The mass number tells us the nucleus contains 19 particles in total. Since 9 of these are protons, the other 10 are neutrons. We call it the mass number because virtually all the mass in an atom comes from the protons and neutrons - electrons weigh about 1/2000th of the mass of a proton or neutron.
Mass Numbers Game
Related questions
